Al2O3, Glass Beads, and Apatitic Abrasive: Comparing Post-Processing Options for 3D-Printed Titanium Medical Implants
For the best chance of successful osseointegration, titanium and titanium-alloy implants need to undergo a surface finishing process. Research has shown that specifically textured surfaces on Ti implants can increase bone-to-implant contact and encourage new tissue growth around the device. The improvements seen with textured implant surfaces holds true whether the implants are produced via 3d printing (additive manufacturing) or through conventional processes such as metallurgical milling or forging.
In particular, additive manufactured implants benefit from post-processing to remove residual beads present on the implant as a result of the 3d printing process. If left untouched, these beads can potentially become dislodged upon packaging, or after implantation.
Image: Himed, LLC
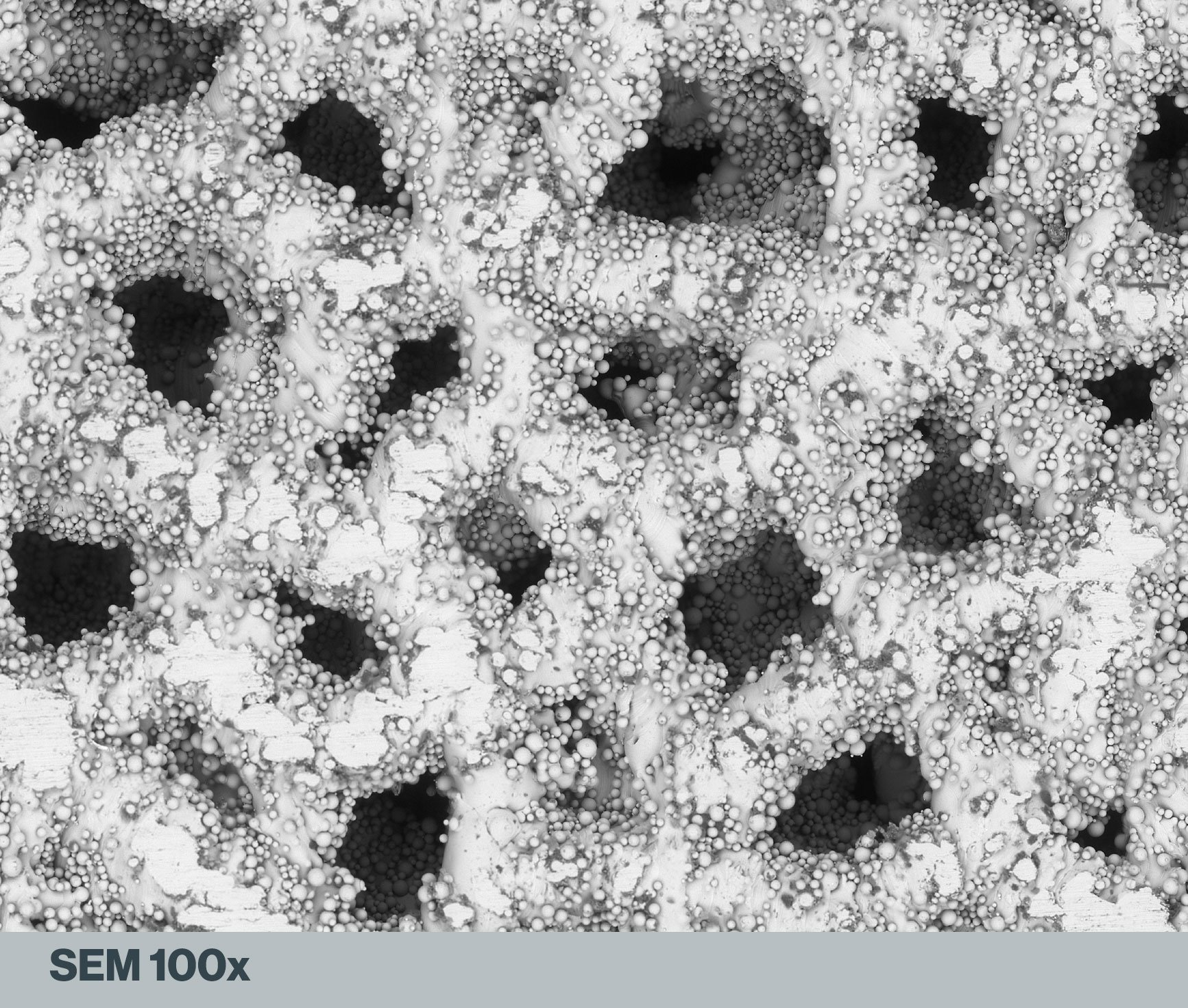
In the scanning electron microscope (SEM) image above of a lattice matrix on a 3D-printed Ti64 spinal spacer, the presence of these loosely adherent residual beads is obvious. They can even be seen on the surfaces of the internal cavities. Other imperfections, in the form of flattened areas on the exterior surface, are likely points at which the implant contacted the printer’s build plate.
While titanium is specifically employed in the design of many medical implants because of its biocompatibility, a detailed understanding of the physiological effects from dislodged Ti particles has yet to be comprehensively studied. Until such research is conducted the logical solution is to apply a safe and effective post-processing methodology to remove the residual beads. Doing so yields aesthetic improvements as well, like eradicating visible layer lines and the surface abnormalities which can result from contact with the build surface. However, there are limited options for such post-processing, and only one that can consistently provide a uniform surface texture to Ti and Ti-alloy implants without introducing different residual contaminants to the surface in the process.
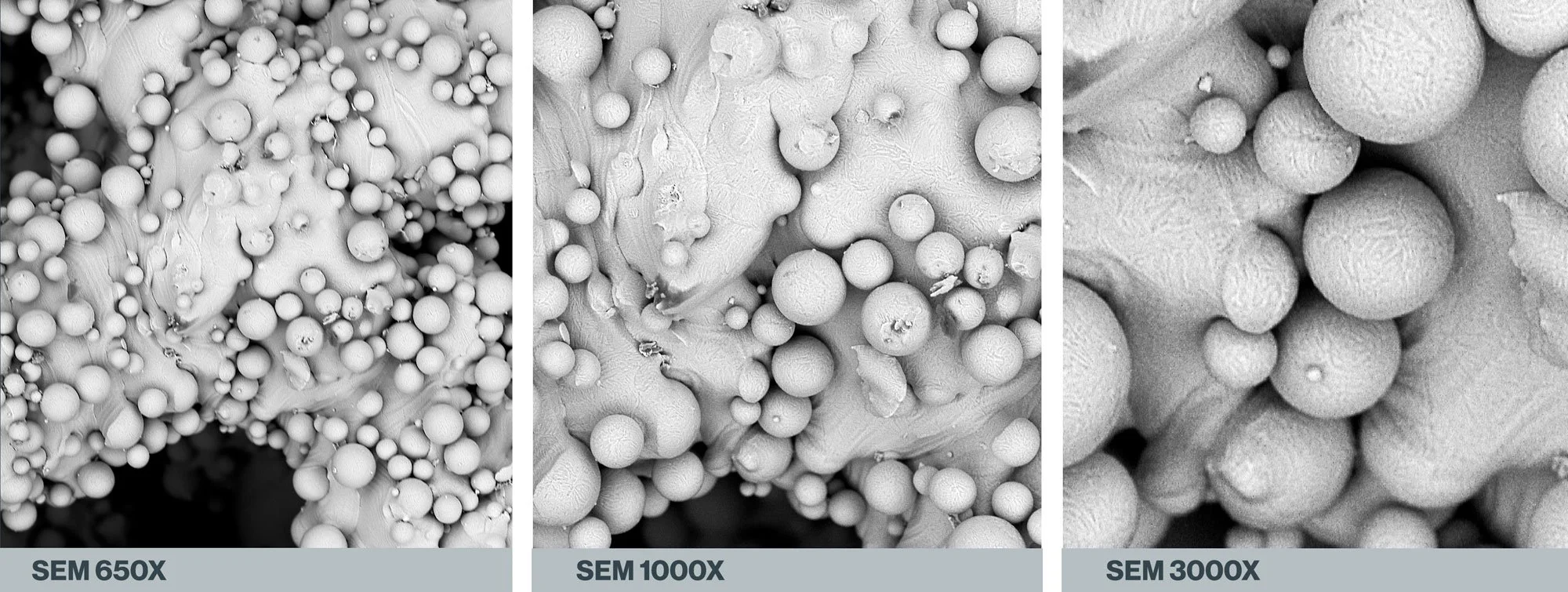
Increasing SEM magnifications of an additive manufactured Ti64 spinal spacer demonstrates that a diversity of scale exists among the residual beads. All images are the property of Himed, LLC.
Adding Benefit, Not Residue, When Post-Processing Additive Manufactured Implants
Abrasive grit blasting has long been a staple of industrial manufacturing applications, with aluminum oxide (or alumina) of differing particle sizes commonly employed on a variety of metals and metal-alloy surfaces because of its extreme hardness (9 on the Mohs scale). However, while conventional grit blasting via aluminum oxide may effectively remove residual beads, these abrasives are notoriously brittle. During application, small pieces or shards will become embedded in the surface of the softer titanium. The embedded particles are insoluble in acids, so removing AI2O3 contamination via ultrasonic bath immersion is not possible.
ALUMINUM OXIDE ABRASIVE BLASTING
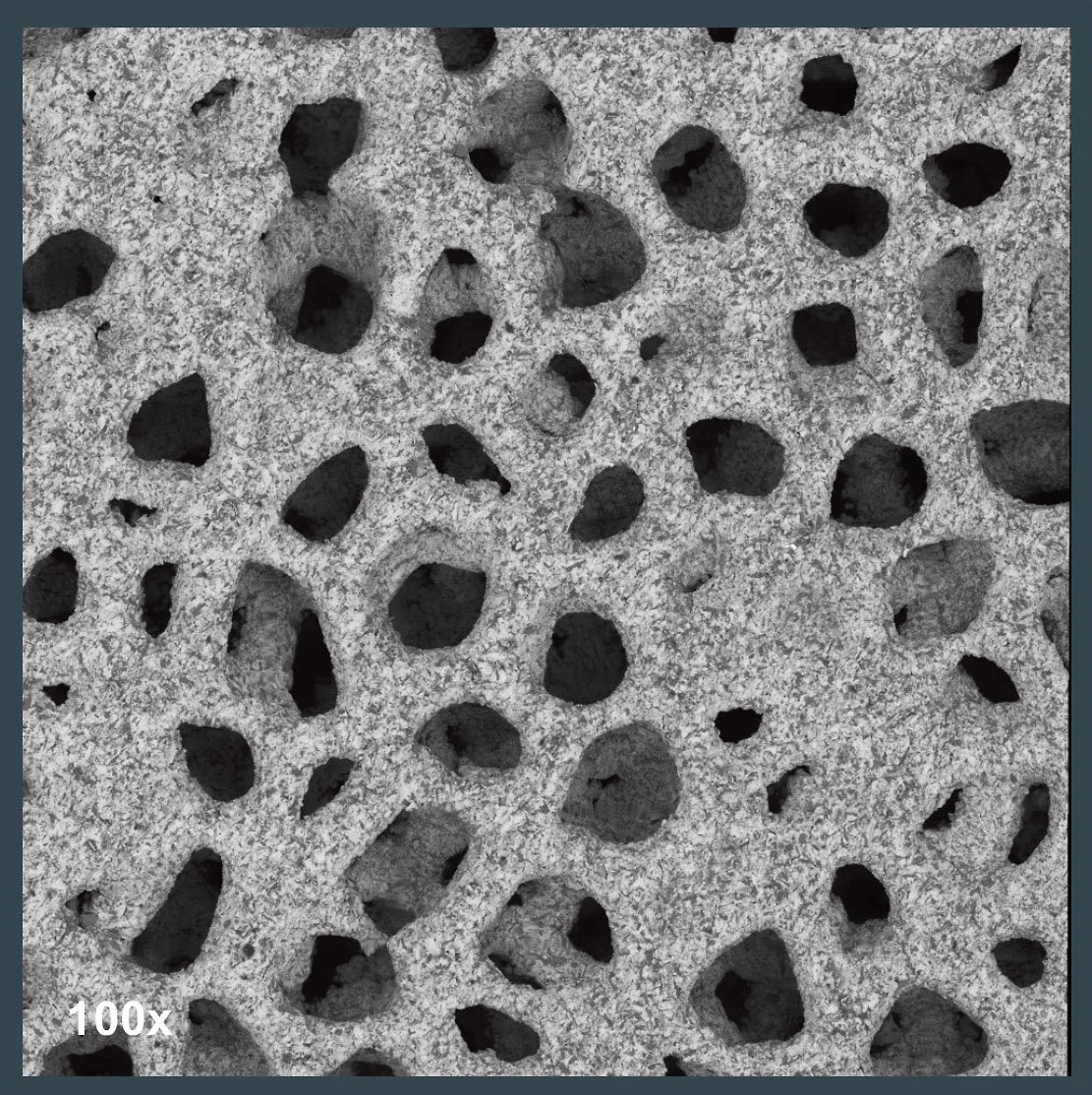
SEM imagery of a 3d-printed Ti64 spinal spacer following abrasive blasting with aluminum oxide grit. Note the dark colored areas across the surface—these are representative of abrasive material embedded in the Ti surface during application. All images are the property of Himed, LLC.
This raises the question: are embedded grains of aluminum oxide any more, or less likely, to shed in vivo as the loosely adherent titanium beads they removed? Further study would be necessary to make an accurate assessment of that possibility.
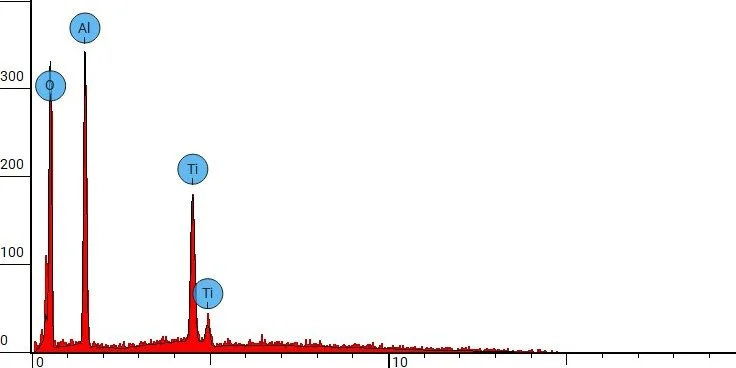
As the SEM images above show, a significant amount of new residual material results from aluminum oxide blasting, and an EDX spot analysis confirms that the implant’s surface can no longer be classified as purely Ti in composition.
Energy-dispersive X-ray spectroscopy (EDX) spot analysis of a 3d-printed Ti64 orthopedic device following abrasive blasting with aluminum oxide grit.
A more recent abrasive blasting option are glass beads. These contain less aluminum oxide overall, but are effective at removing surface abnormalities from a 3d-printed Ti or Ti-alloy implant, even with a much lower Mohs rating of approximately 5.5.
GLASS BEAD ABRASIVE BLASTING
SEM magnification of a 3d-printed Ti64 spinal spacer following post-processing with glass beads. Darker areas on the surface indicate residual contaminants that remain following post-processing. All images are the property of Himed, LLC.
Nevertheless, like conventional aluminum oxide abrasives, the glass beads can shatter upon contact with the implant surface—thereby introducing new particulates into the Ti which cannot be fully eradicated through passivation. Spot test analysis via EDX of these particles predictably reveal less aluminum but more silicon. And the reduction of pure Ti surface area due to the addition of glass bead fragments.
Energy-dispersive X-ray spectroscopy (EDX) spot analysis of a 3d-printed Ti64 orthopedic device following abrasive blasting with glass beads.
Fortunately, Himed developed a micro-abrasive material nearly 30 years ago that is ideally suited to post-processing the 3d-printed implants of today. It is completely soluble, and leaves behind no trace residue following ASTM F86 passivation. Originally conceived to provide a uniformly textured surface to Ti dental implants prior to a coating of hydroxyapatite, this apatitic abrasive offers an ideal solution for the post-processing of 3d-printed implants of all shapes and sizes.
Biocompatible Post-Processing That Adds an Optimized Surface Texture for Osseointegration
MCD Apatitic Abrasive is a calcium phosphate abrasive, principally composed of hydroxyapatite and tricalcium phosphate. It is formulated to be as hard as possible, and can obtain a Mohs hardness rating of up to 5. Available in a wide variety of sizes ranging from 425 μm, the appropriate granular size may be chosen so that media can effectively reach internal porous surface cavities to create a uniform surface roughness (Ra). While notably less hard than aluminum oxide, it is only slightly softer than many commercially available glass beads. Nevertheless, it is still capable of refining Ti and Ti-alloy within a Ra range of 1.0μm–3.2μm through adjustments in particle size, blast pressure, duration, and distance. Furthermore, its gentler contact with the implant surface means that critical tolerances and shape details within the original design are preserved.
APATITIC ABRASIVE BLASTING
SEM sequence demonstrating the post-passivation results of a MATRIX MCD application of <53μm apatitic abrasive. Particle size impacts the relative smoothness of the refined surface, with larger MCD of 425–180μm yielding a rougher surface topography. All images are the property of Himed, LLC.
As evidenced in the SEM sequence above, the implant surface following micro-abrasive blasting with MCD apatitic abrasive presents a textured Ti topography on both the external and internal implant surfaces. Unlike with aluminum oxide grit or glass beads, there is no evidence of any residual contaminant after passivation that might inhibit biological fixation or shed in vivo.
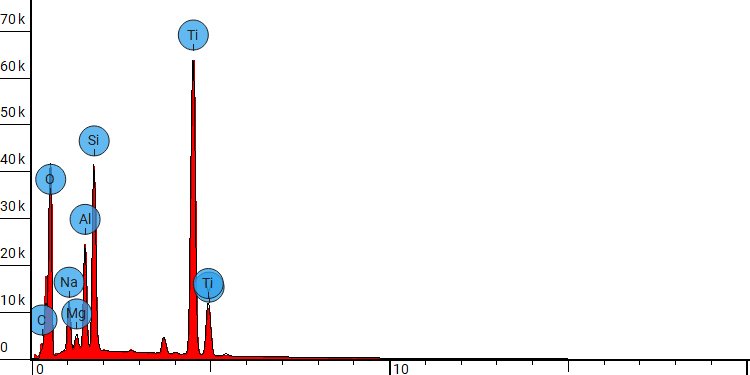
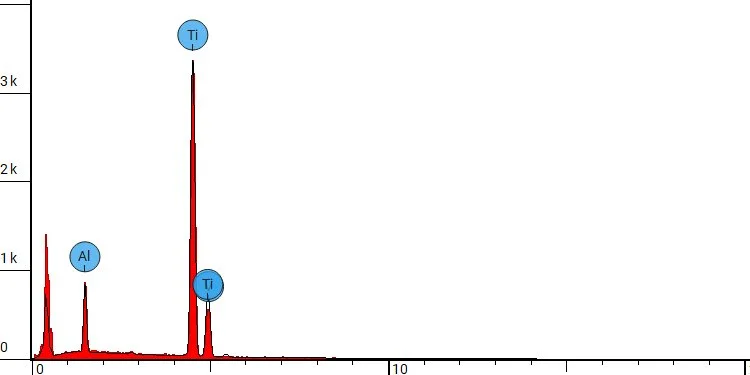
Energy-dispersive X-ray spectroscopy (EDX) spot analysis of a 3d-printed Ti64 orthopedic device following apatitic abrasive blasting with Himed’s proprietary MATRIX MCD® process.
Over three decades, Himed has refined the application of MCD apatitic abrasive to meet the exacting standards of a wide variety of orthopedic and dental implant designs. Comprehensive analysis of the post-processing results possible via MCD apatitic abrasive are available as free white paper downloads via the button below.
There is no question that the emergence of additive manufacturing technologies into the medical and orthopedic fields has been transformative, advancing device intricacy and performance while offering patient-specific solutions. Himed is pleased to offer a solution to the complex challenges of post-processing this new generation of intricate 3d printed devices…even if we arrived at that solution a quarter-century before 3d printing was a viable technology!