
Himed and Lithoz Announce Launch of New Bioceramics Center of Excellence™
Himed and Lithoz are pleased to announce the launch of a new Bioceramics Center of Excellence™ (BCoE) at Himed's New York headquarters. The BCoE will offer a holistic approach to bioceramics R&D for medical device manufacturers, integrating various analytical services to support rapid prototyping and device customization.
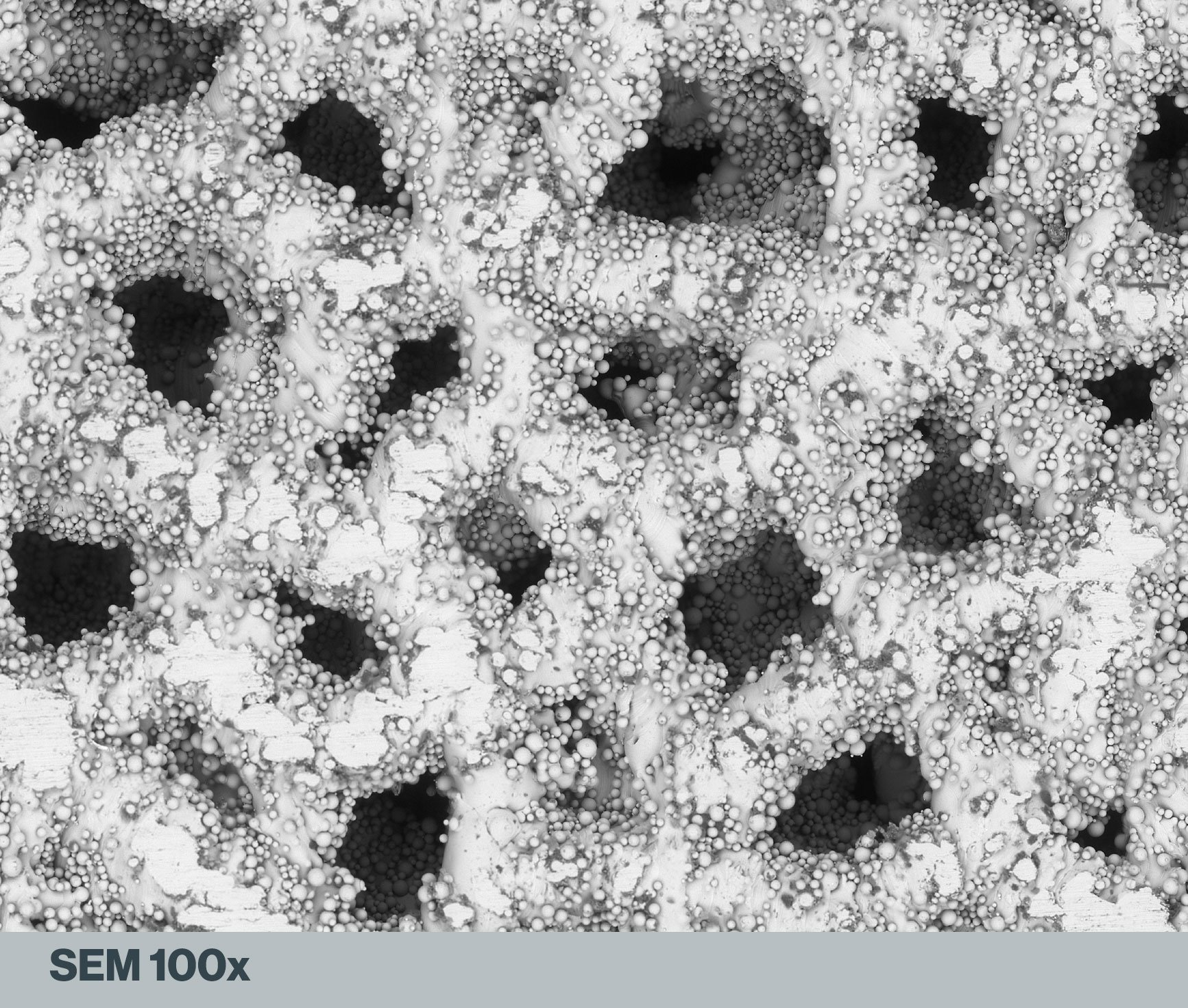
Al2O3, Glass Beads, and Apatitic Abrasive: Comparing Post-Processing Options for 3D-Printed Titanium Medical Implants
For the best chance of successful osseointegration, titanium and titanium-alloy implants need to undergo a surface finishing process. Research has shown that specifically textured surfaces on Ti implants can increase bone-to-implant contact and encourage new tissue growth around the device.
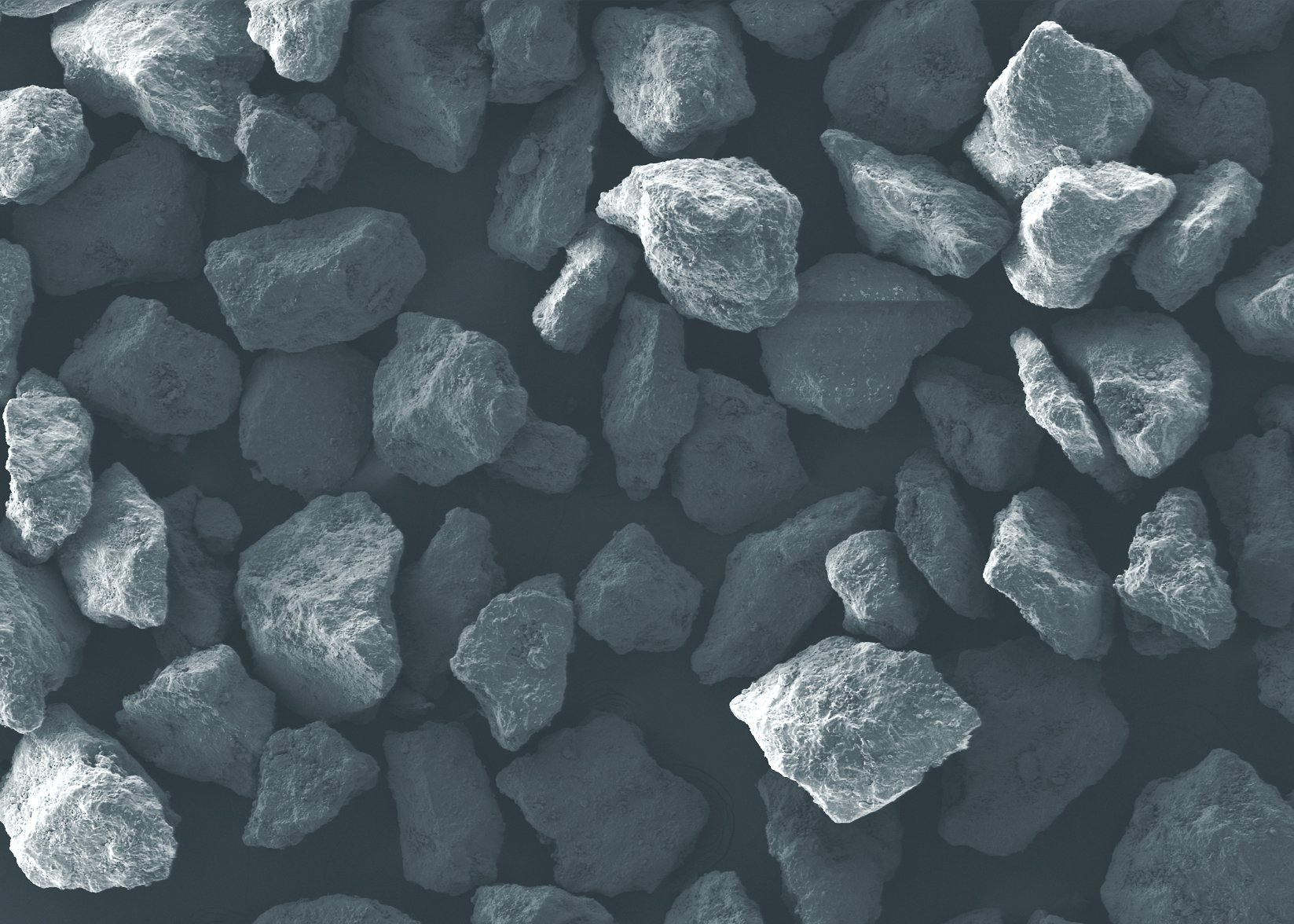
An Ideal Post-Processing Solution for Additive-Manufactured Medical Devices
Few things have been as potentially revolutionary for the medical device industry as 3D printing. The process allows implant manufacturers to design and produce innovative products quickly and efficiently. But additive manufacturing does introduce a few specific issues medical implant designers and fabricators must take into account.

White Paper on Finishing 3D Printed Titanium Implants
While 3D printing has transformed the rapid production of intricate implantable devices, post-process finishing is often a necessity for critical improvements to the aesthetic appearance and overall functionality. MATRIX MCD® Apatitic Abrasive micro-blasting presents an efficient and highly effective option!
This whitepaper, published by ORTHOWORLD® in the latest BONEZONE/OMTEC eNewsletter, reviews multiple case studies to demonstrate these advantages.
